- Home
- Fundamentals of graphite
Fundamentals of graphite
Structure
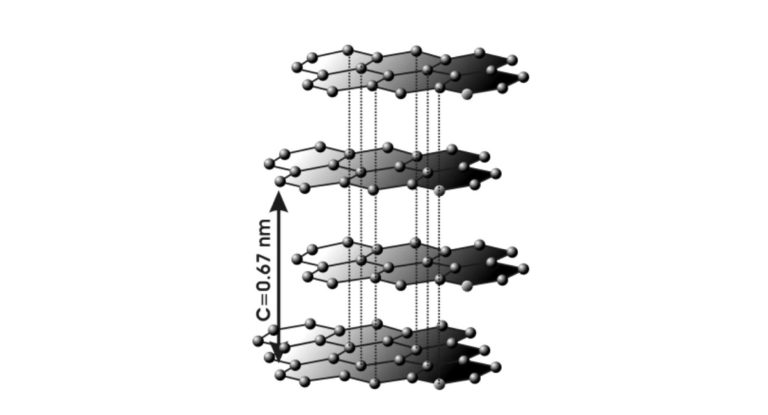
Graphite crystallizes in parallel layers, known as basal planes. Within these planes, the bonding energy between carbon atoms is 4.3 electron volts, whereas the bonding energy between the planes is only 0.07 electron volts. This extreme directional dependence of bonding forces results in a distinct anisotropy of graphite’s mechanical, electrical, and thermal properties:
- Easy cleavage of pure graphite along the basal planes, while showing significantly higher strength along the crystal layers.
- Thermal and electrical insulation perpendicular to the basal planes, but almost metallic conductivity along the planes.
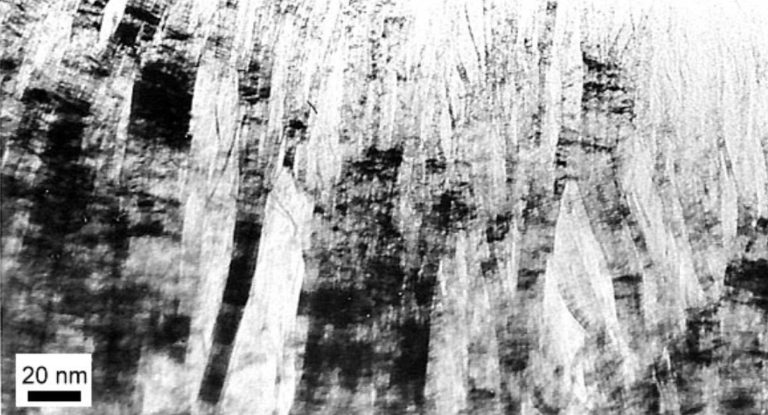
If the basal planes have no fixed correlation to one another, the structure is referred to as “turbostratic” carbon. Transmission electron microscopy reveals the stacking of basal planes in graphite. The overlap of tilted stacks creates Moiré patterns, though the 0.34-nanometer basal plane spacings are not always clearly resolved.
In glass-like carbon, the planes are not arranged parallel like the pages of a book, but rather randomly crumpled like crushed paper. This carbon is hard and isotropic, similar to glass, hence its name. Through specific processing techniques, such as stretching polymer precursors and subsequent graphitization, it is possible to orient the layers in the fiber direction, resulting in high-strength carbon fibers.
Fullerenes and nanotubes consist of just one basal plane, which is curved into a sphere (fullerenes) or a tube (carbon nanotubes). These forms are closely related to graphite, and additional layers can grow in an onion-like structure, creating soot-like powders.
Occurrence
Production
Applications
Graphite is widely used in various industries, including:
- Pencil leads
- Black printing inks (as carbon black)
- Rubber filler in tires (as carbon black)
- Lubricants
- Bearings and seals
- Enhancing electrical conductivity
- Carbon brushes in electric motors
- Compressed electrodes for electrical power transmission
- Electrodes in arc lamps
- Crucibles for high-temperature applications
- High-temperature furnace linings (in reducing atmospheres)
- Nuclear reactor control
- Activated carbon for gas, liquid, and particle adsorption
- Monochromators in X-ray diffractometers

Do you have any questions?
We look forward to your message or call:
Phone: +49 (0) 228 – 410300
Fax: +49 (0) 228 – 4103070
